Molecular Carrier-Assisted Self-Assembly of st-PMMA Helical Complexes for Fluorescence Sensing of Nitroaromatics in Aqueous Medium
Yu-HaoWang,Wen-TsungTseng, and Kuan-Yi Wu*
ABSTRACT
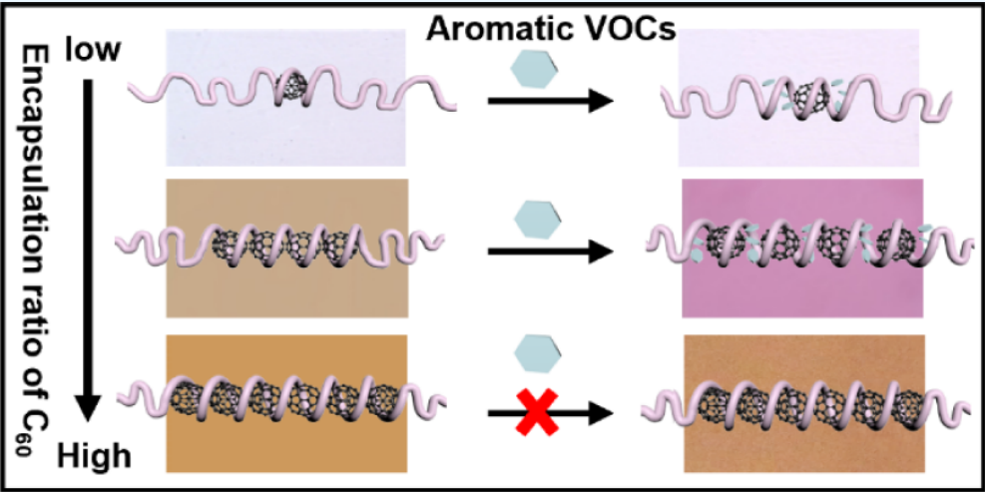
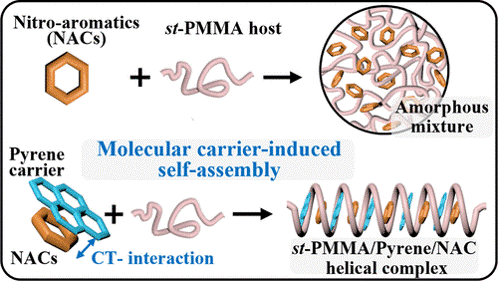
In nature, the cooperative binding of enzyme, coenzyme, and substrate forms a functional ternary complex that activates catalytic activity essential for biological functions. Inspired by this mechanism, we present a supramolecular strategy employing pyrene as a molecular carrier to facilitate the encapsulation of nonbinding nitroaromatic compounds (NACs) into the helical structure of syndiotactic poly(methyl methacrylate) (st-PMMA) via charge-transfer (CT) interactions. Structure characterization revealed that NACs and st-PMMA only form amorphous mixtures due to the weak binding affinity. In contrast, pyrene guests can coassemble with st-PMMA host to form helical complexes. Upon adding NACs to the st-PMMA/pyrene system, CT complexation between pyrene and NACs occurs readily in solution. Given the specific molecular recognition between pyrene and the st-PMMA host, pyrene can act as the carrier, enabling the pyrene-tagged NACs to be subsequently encapsulated within the st-PMMA helix during the transition to the solid state. Spectroscopic analyses confirm that the nonradiative CT complex of pyrene/NACs suppresses pyrene excimer formation in the st-PMMA helix and leads to the fluorescence quenching. Notably, owing to the dynamic nature of molecular carrier-induced self-assembly, the transparent st-PMMA/pyrene complex films demonstrate effective detection of explosive NACs in aqueous medium. Therefore, these findings establish a cooperative self-assembled strategy for modulating inclusion behavior in helical polymers, offering new avenues for advanced sensing platforms and precision-controlled molecular encapsulation.
KEYWORDS: molecular recognition, cooperative self-assembly, inclusion complex, stereoregular polymer, nitro-aromatic compounds.